| « La Lune s'éclipse le 28 septembre 2015 | NON au cinéma 3D avec lunettes ! » |
16/04/2015
Ion sensitive electrodes preparation
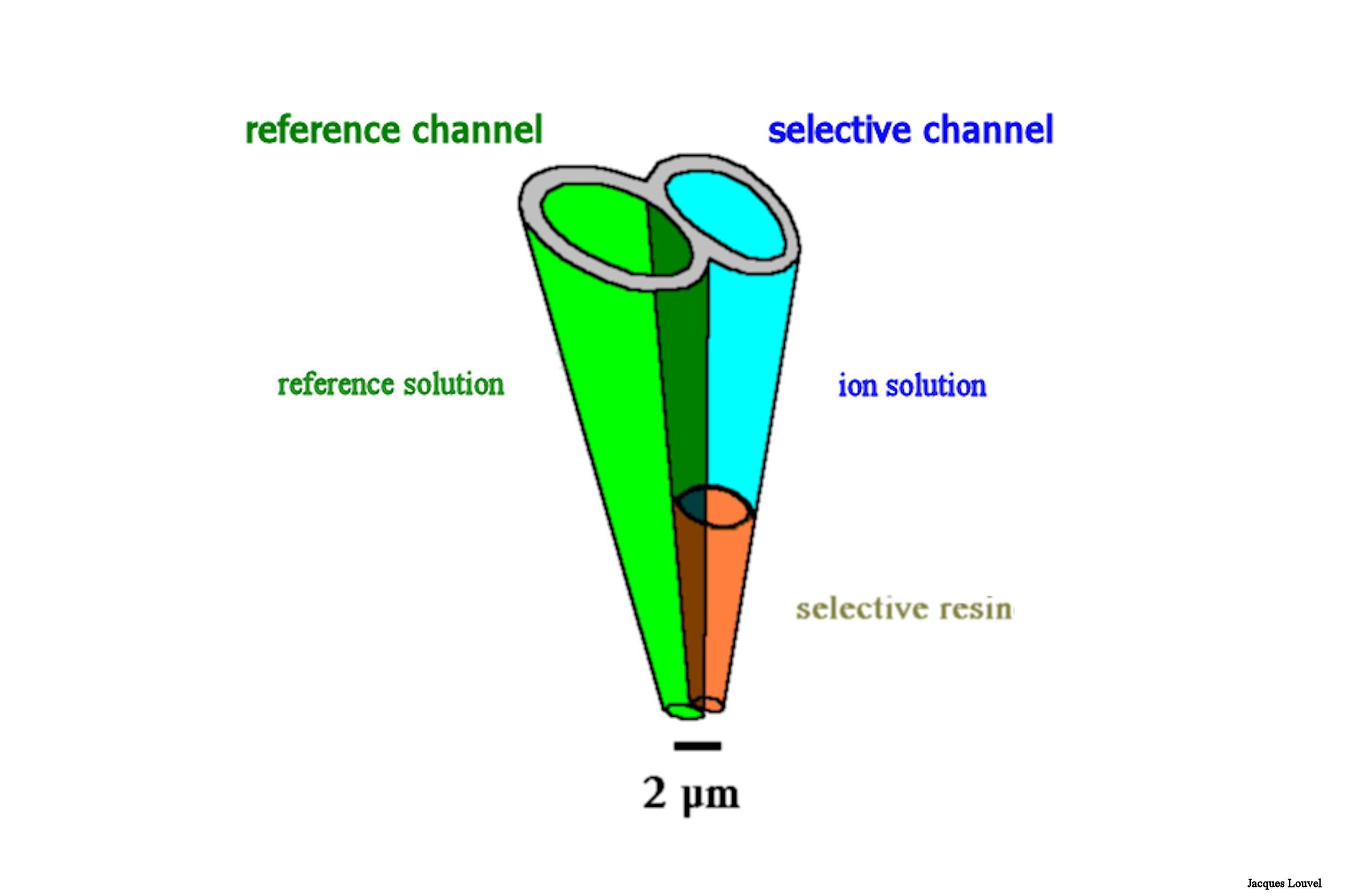
We have now our double barrel glass micropipette. We have to fill it. One barrel will be used to form a standard reference. We have no particular problem with this one. We just fill it with the proper electrolyte for extracellular recordings and we forget about it. However, the so called "selective" or "sensitive" barrel needs some more equipment and skills to be filled.
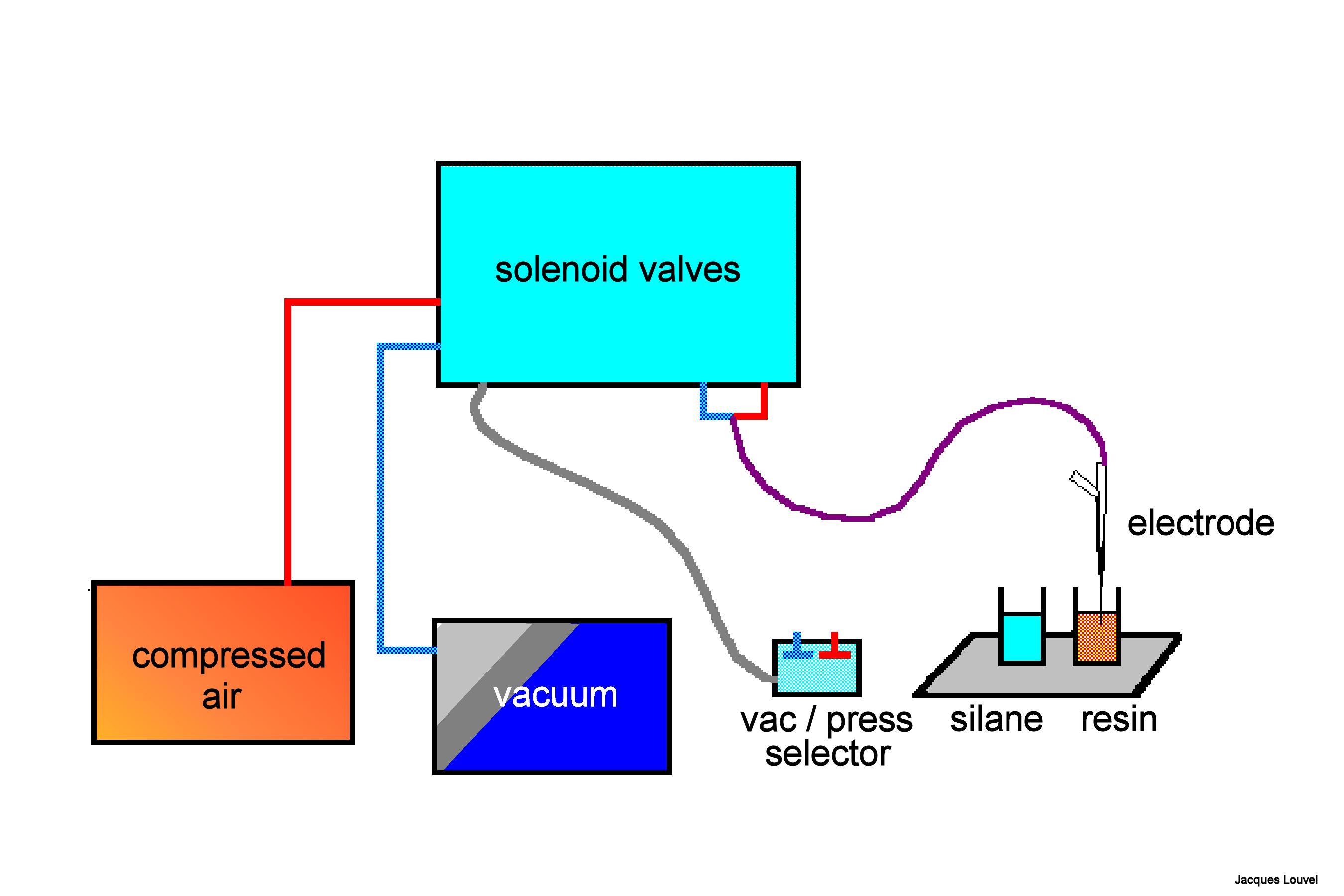
Below is a schematic drawing of the setup used to fill the "ionic side" of our micropipette.
The principle is to suck up the resin into the tip. So a source of vacuum connected to the micropipette is needed (i.e. a vacuum pump). But there is one more problem. The resin is highly hydrophobic while the glass is hydrophilic. The solution is to treat the inner surface of the tip in order to render it hydrophobic too. This will be achieved by coating the glass with a silane derivative. In practice, we shall have to suck the silane in and then push it out. For this we need also a source of pressure connected to the pipette and a possibility to alternate between pressure and vacuum.
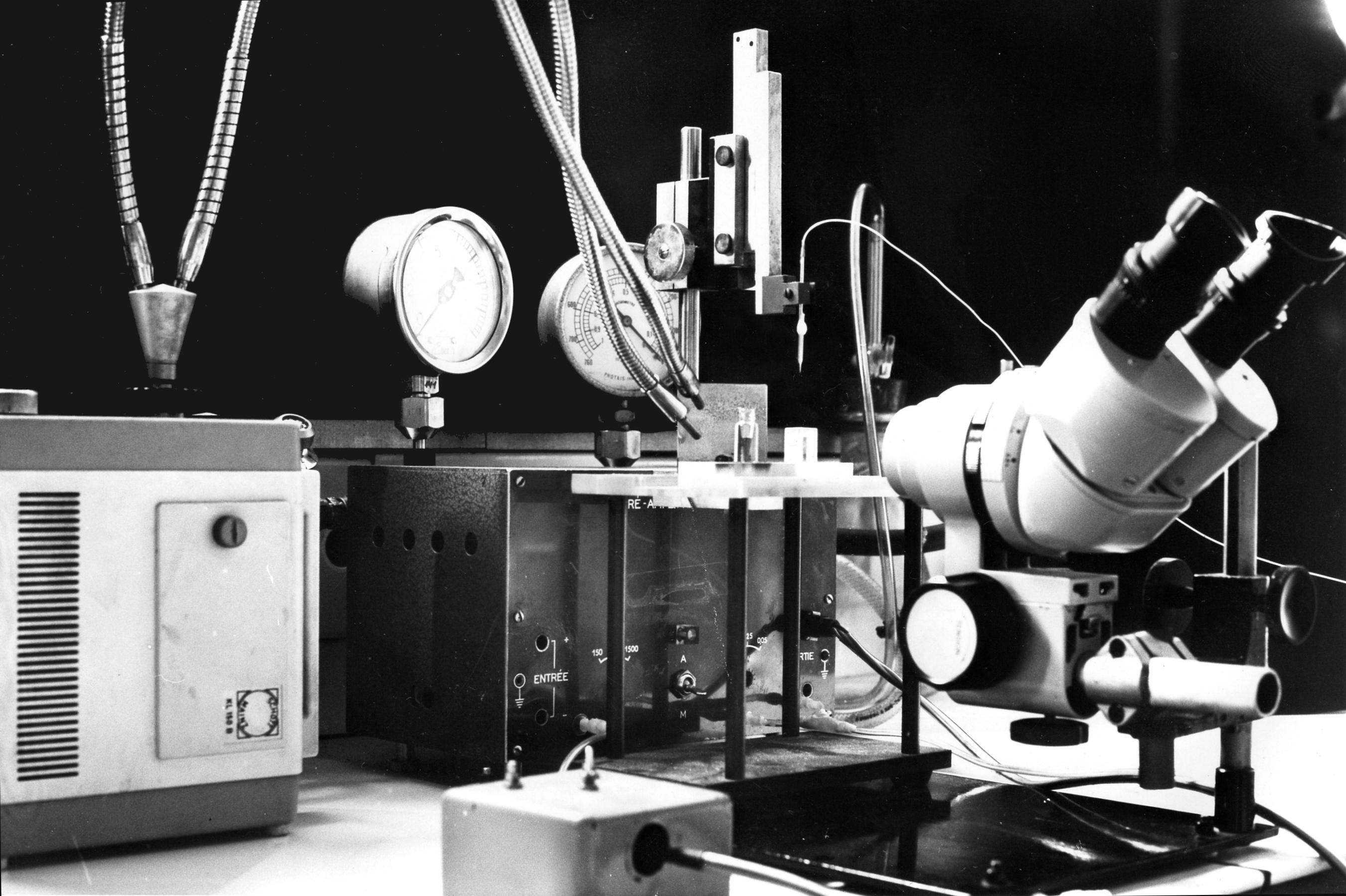
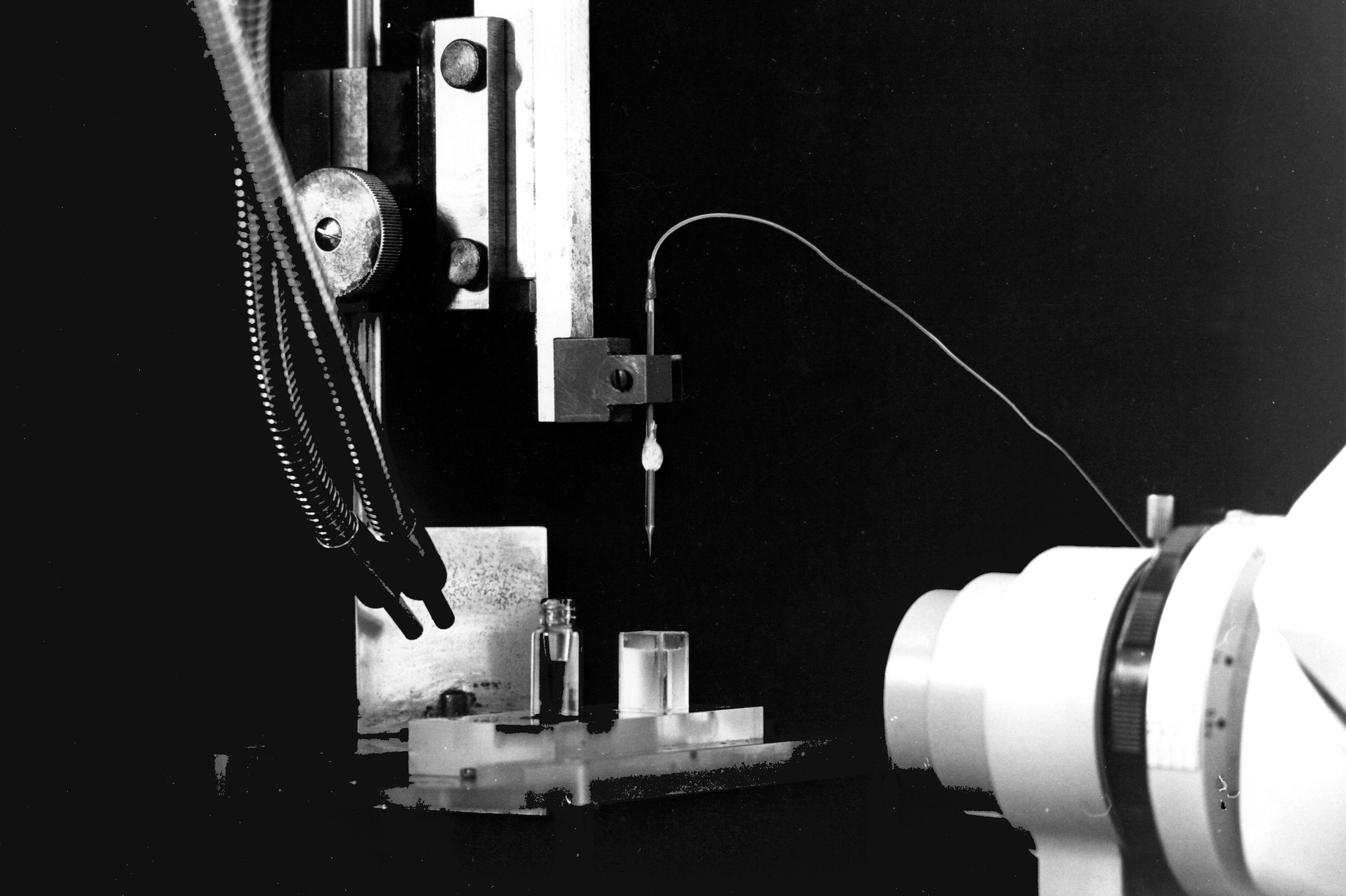
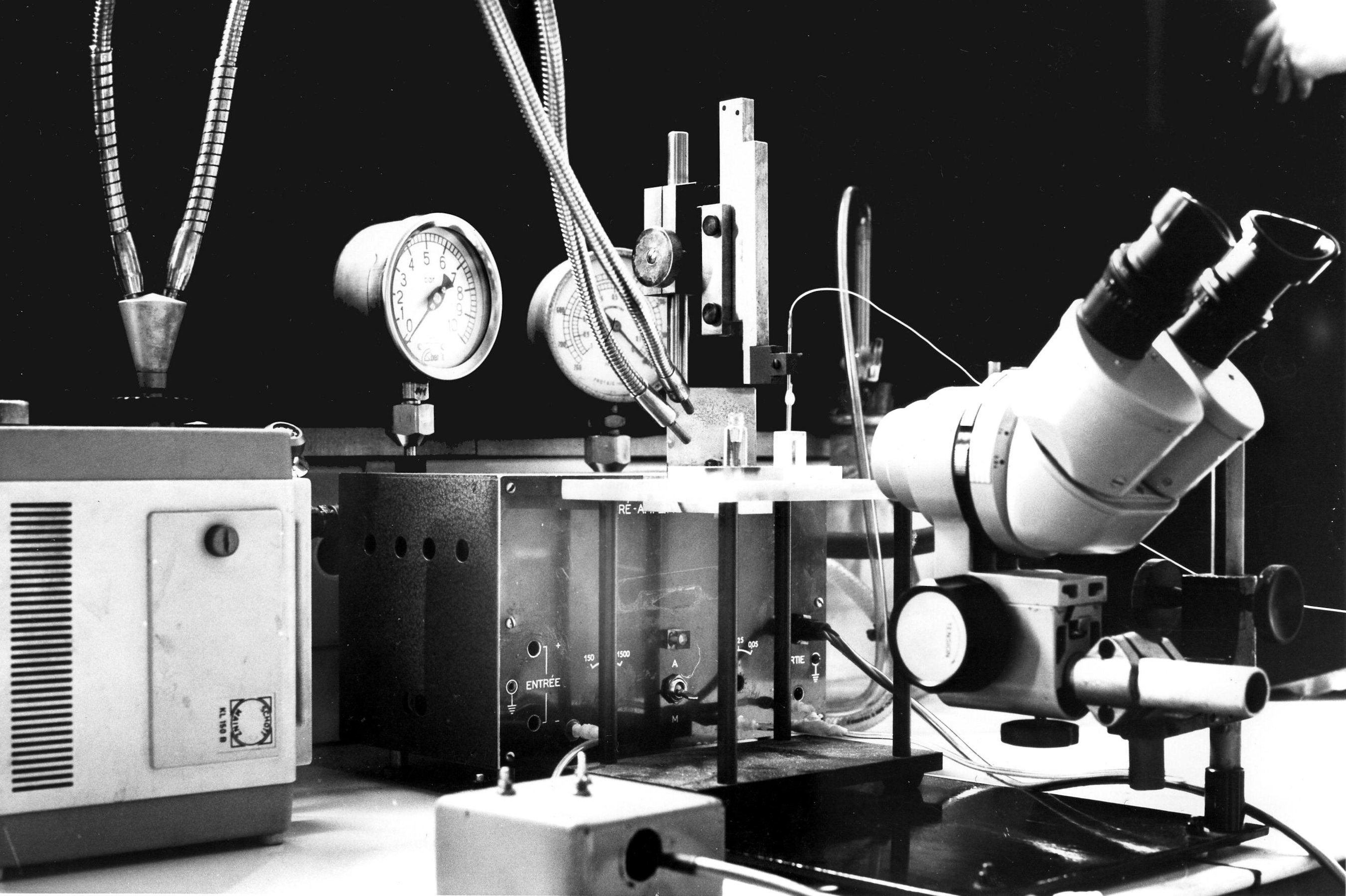
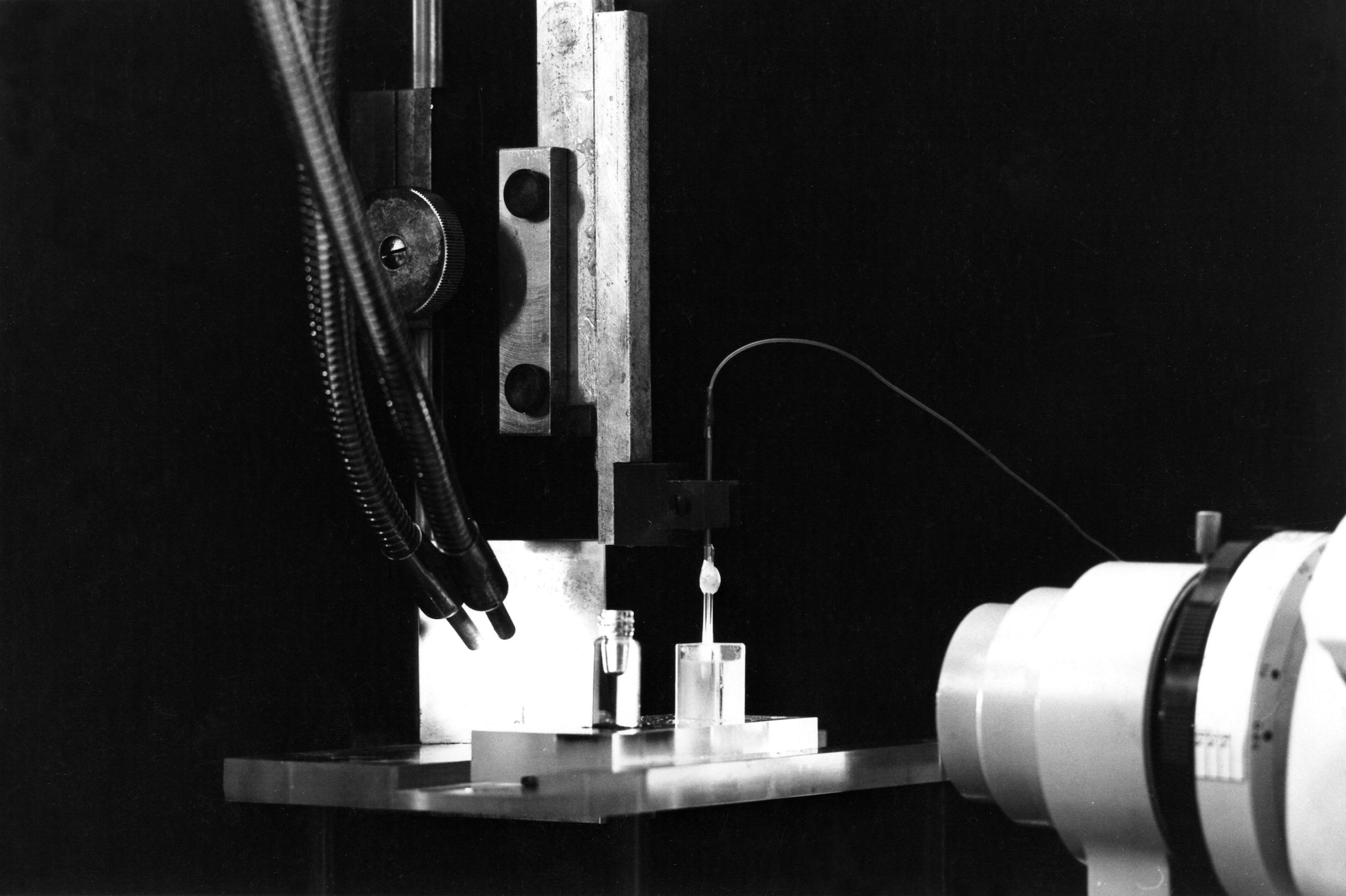
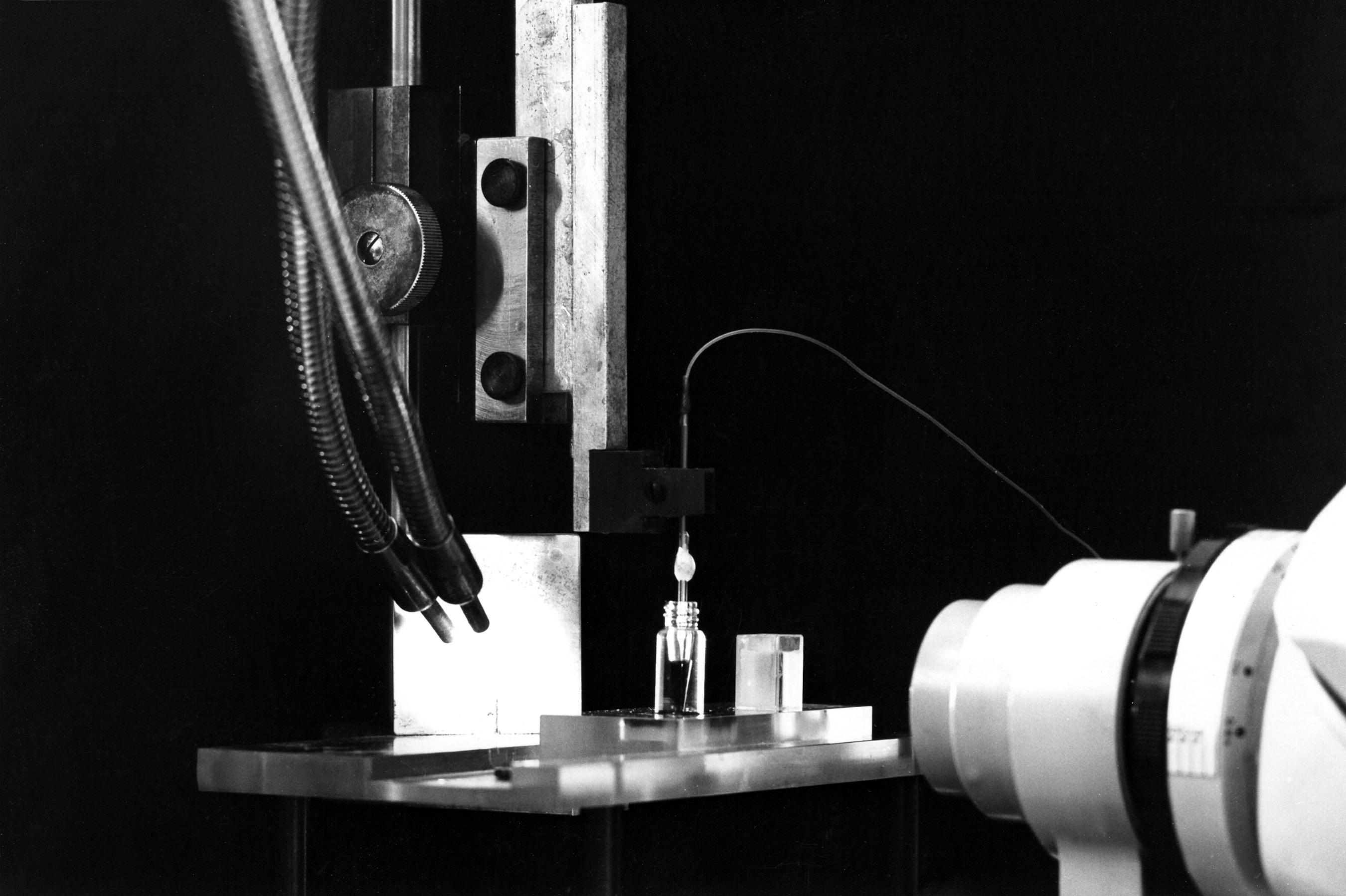
We need a binocular microscope (on the right in the photo) to control the extent of the coating. This will not be easy since both the filling solution of the micropipette and the silane solution are transparent. We shall have to rely on the meniscus which forms between the two solutions. So a good light is necessary (on the left in the photo). The photos show the rectangular glass cell with parallel sides which contains the silane and the vial which contains the resin. The photos also show the pipette fixed on a micro manipulator. A tubing is attached to the pippette. It is used to bring pressure or vacuum. The other end of this tubing is connected to the box in the background which contains the solenoid valves controlling the pressure or vacuum. The switches on the left of the binocular microscope control the opening of the valves.
When the coating is done (several pull and push are necessary), we insert the pipette tip into the resin vial. Pulling the resin up into the tip should be possible - although not easy - in the silane coated portion. The final result should be a column of resin not more than a few ten of microns in height. The higher the column of selective resin, the slower will be the response of the electrode. But if the column is too small, then there is an increased risk to loose it when moving the pipette inside the brain tissue.
The filled "ionic" micropipette, must be calibrated. Please see next page (click here or select page 4)
No feedback yet
Form is loading...